
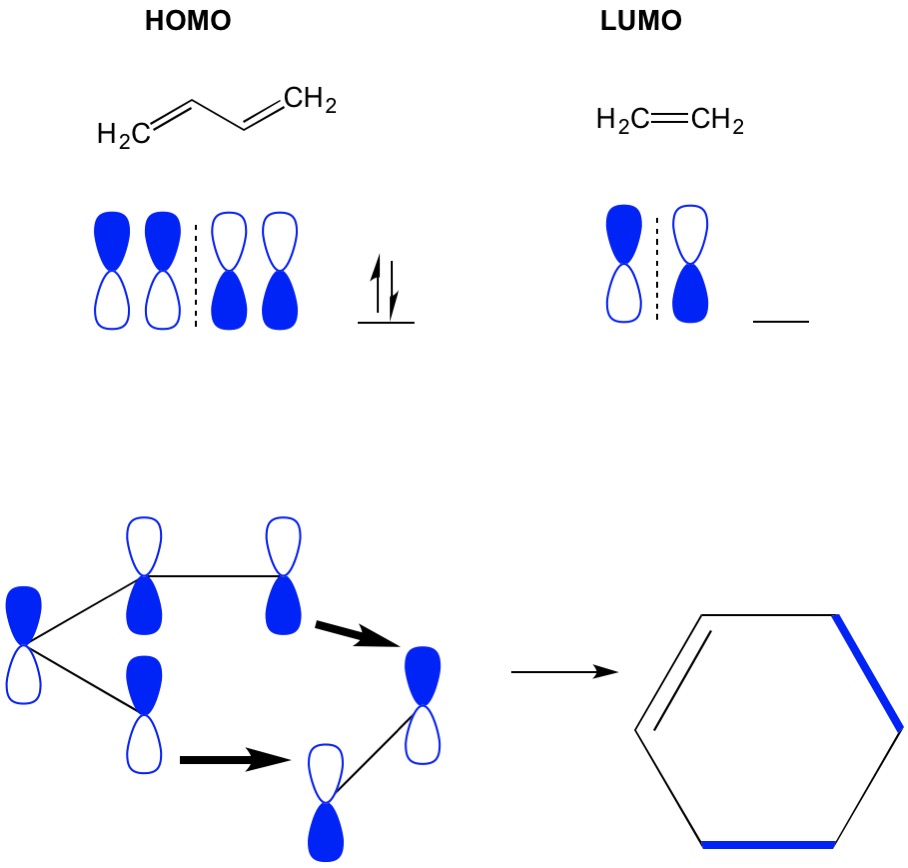
Here to investigate this, cyclobutadiene 2 is reacted with acetylene and ethylene, two examples of unactivated dienophiles. In addition, the presence of the highly electron-withdrawing pentafluorophenyl group on the cyclobutadiene ring could lead to unique Diels–Alder reactivity, such as inverse electron-demand Diels–Alder cyclization. In fact, tetrahedrane 1 can be efficiently photochemically isomerized into pentafluorophenyltris(trimethylsilyl)cyclobutadiene ( 2), which is a rare example of cyclobutadiene with two different substituents 30. However, we recently reported that tetrahedranes can also be used as the precursor to synthesize new types of cyclobutadienes. We have reported a variety of silyl-substituted tetrahedranes starting from this single precursor, including pentafluorophenyltris(trimethylsilyl)tetrahedrane ( 1), in recent years 26, 27, 28, 29. However, the activation of benzene and its use as a diene or dienophile remain as challenges in organic chemistry.Ĭyclobutadienes are a valence isomer of tetrahedranes and often serve as a convenient precursor in the synthesis of these highly strained cage compounds, particularly tetrakis(trimethylsilyl)tetrahedrane. As a rather peculiar case, 1,4-addition of benzene to a dihydrocyclopentindene diradical is also reported 25. Alternatively, exceptionally strong dienophiles or dienes under severe conditions (for example, high temperature and pressure) will undergo Diels–Alder cyclization with benzene 15, 16, 17, 18, 19, 20, 21, and reactive unsaturated heavier main group analogues of an alkene or alkyne make adducts with arenes under mild conditions 22, 23, 24. Currently, there are only a limited number of options to activate benzene sufficiently, including coordination of a transition metal complex to benzene 10, naphthalene with small resonance energy 11 and the use of benzene derivatives with a sterically induced strained geometry 12, 13, 14. The limited reactivity of this π system is because of the enormous stability afforded by aromaticity, which can only be overcome under certain conditions 18. Only a limited number of examples of the Diels–Alder reaction involving benzene derivatives have been reported 10, 11, 12, 13, 14, 15, 16, 17. In contrast to cyclobutadiene, benzene rarely participates in Diels–Alder reactions. However, aside from the dimerization process, cyclobutadienes almost exclusively react as electron-rich dienes. Even isolable cyclobutadienes, with bulky substituents, undergo Diels–Alder cyclization with a variety of reagents 6, 7, 8, 9. In fact, cyclobutadienes with small substituents are sufficiently reactive to dimerize via a Diels–Alder reaction and can also be trapped with a large variety of dienophiles 3, 4, 5. The archetypal example of cyclobutadiene reactivity is the Diels–Alder reaction. Cyclobutadiene, with four π electrons, is anti-aromatic and has a planar, monocyclic structure with bond alternation and high reactivity, which is attributed to its high-lying highest occupied molecular orbital (HOMO) and low-lying lowest unoccupied molecular orbital (LUMO). Benzene, with six π electrons, has special characteristics attributed to aromaticity, such as equivalent C–C bonds, inertness towards addition reactions and so on. According to Hückel’s rule, monocyclic systems containing π electrons are aromatic, while systems containing 4 n π electrons are antiaromatic 1, 2. Aromaticity is one of the fundamental concepts of organic chemistry.


 0 kommentar(er)
0 kommentar(er)
